There are number of ways to measure the cost of energy when you are comparing, say solar photovoltaic vs. coal-fired power for example. When comparing simple cost per megawatt-hour over a 30-year power plant life-cycle, we are now at or near the point in the vast majority of locations around the world where solar and wind energy installations are a better value for power production over time than are non-renewable options such as coal, petroleum, nuclear, or even natural gas. This is not even taking an accounting of externalities and risks. But a major issue that is keeping solar and wind from dominating new power construction is the issue of dispatchability or peak-load potential. Dispatchable generation is that which can be turned off or on at any time and at nearly any capacity to meet fluctuations in demand. When there is a power outage for some reason, there needs to be a way to bring new sources quickly online.
Solar and wind are both making strides in addressing their variability when it comes to base load supply, with increased flexibility in the transmission grids and with some amount of inherent energy storage to modulate for the intermittent nature of the winds and the sun. For example, solid state thermal storage and sodium thermal storage are providing larger plants with the ability to produce a nearly constant supply of energy to match the performance of a nuclear plant. Reverse-hydro offers another, albeit geographically limited, option for large-scale storage.
But we are now on the verge of also seeing a battery technology revolution that will allow for on demand access to stored renewable energy capacity that will make it possible to imagine a more robust and flexible 100% renewable energy infrastructure that can free us from even the “bridge technology” of natural gas-fired peaking plants.

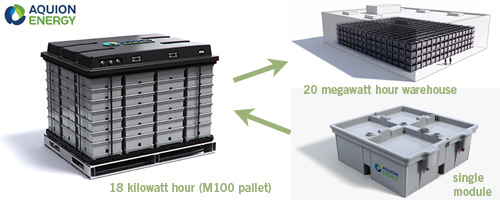
There are a number of recent breakthrough technologies that promise mass energy storage with little environmental impact, such as Pittsburgh-based Aquion Energy’s modular salt water batteries (featured above: click the image for more information). And last week, research at Harvard University has shown proof of concept for a new and efficient family of organic flow batteries that use harmless and abundant organic materials similar in chemistry to the molecules that store energy in plants (in this case, rhubarb). From the Harvard article:
Flow batteries store energy in chemical fluids contained in external tanks—as with fuel cells—instead of within the battery container itself. The two main components—the electrochemical conversion hardware through which the fluids are flowed (which sets the peak power capacity), and the chemical storage tanks (which set the energy capacity)—may be independently sized. Thus the amount of energy that can be stored is limited only by the size of the tanks. The design permits larger amounts of energy to be stored at lower cost than with traditional batteries. […]
To store 50 hours of energy from a 1-megawatt power capacity wind turbine (50 megawatt-hours), a possible solution would be to buy traditional batteries with 50 megawatt-hours of energy storage, but they’d come with 50 megawatts of power capacity. Paying for 50 megawatts of power capacity when only 1 megawatt is necessary makes little economic sense.
For this reason, a growing number of engineers have focused their attention on flow battery technology. But until now, flow batteries have relied on chemicals that are expensive or difficult to maintain, driving up the energy storage costs.
The active components of electrolytes in most flow batteries have been metals. Vanadium is used in the most commercially advanced flow battery technology now in development, but its cost sets a rather high floor on the cost per kilowatt-hour at any scale. Other flow batteries contain precious metal electrocatalysts such as the platinum used in fuel cells.
The new flow battery developed by the Harvard team already performs as well as vanadium flow batteries, with chemicals that are significantly less expensive, and with no precious metal electrocatalyst.
“The whole world of electricity storage has been using metal ions in various charge states but there is a limited number that you can put into solution and use to store energy, and none of them can economically store massive amounts of renewable energy,” Gordon said [Roy G. Gordon is Harvard’s Thomas Dudley Cabot Professor of Chemistry and Professor of Materials Science]. “With organic molecules, we introduce a vast new set of possibilities. Some of them will be terrible and some will be really good. With these quinones we have the first ones that look really good.”
These new battery technologies are going to increase the ability of distributed clean energy microgrids to impact on the existing power landscape in all sorts of ways. With the inevitable application of salt water and flow batteries into the energy infrastructure of our future electrical grids, the questions are:
How will these storage units display themselves in our cityscapes? Can we think creatively about how to integrate these modules into our architecture? Are there ways in which batteries can merge in functionality with state-changing insulation materials? Are there opportunities to create beautiful parks and transport corridors while safely lining our landscapes with energy storage devices? And of course, what role does public art have in the manifestation of energy storage infrastructures?
